Oncology/cancer research
Cancer Clinical Research Program at Salinas Valley Health
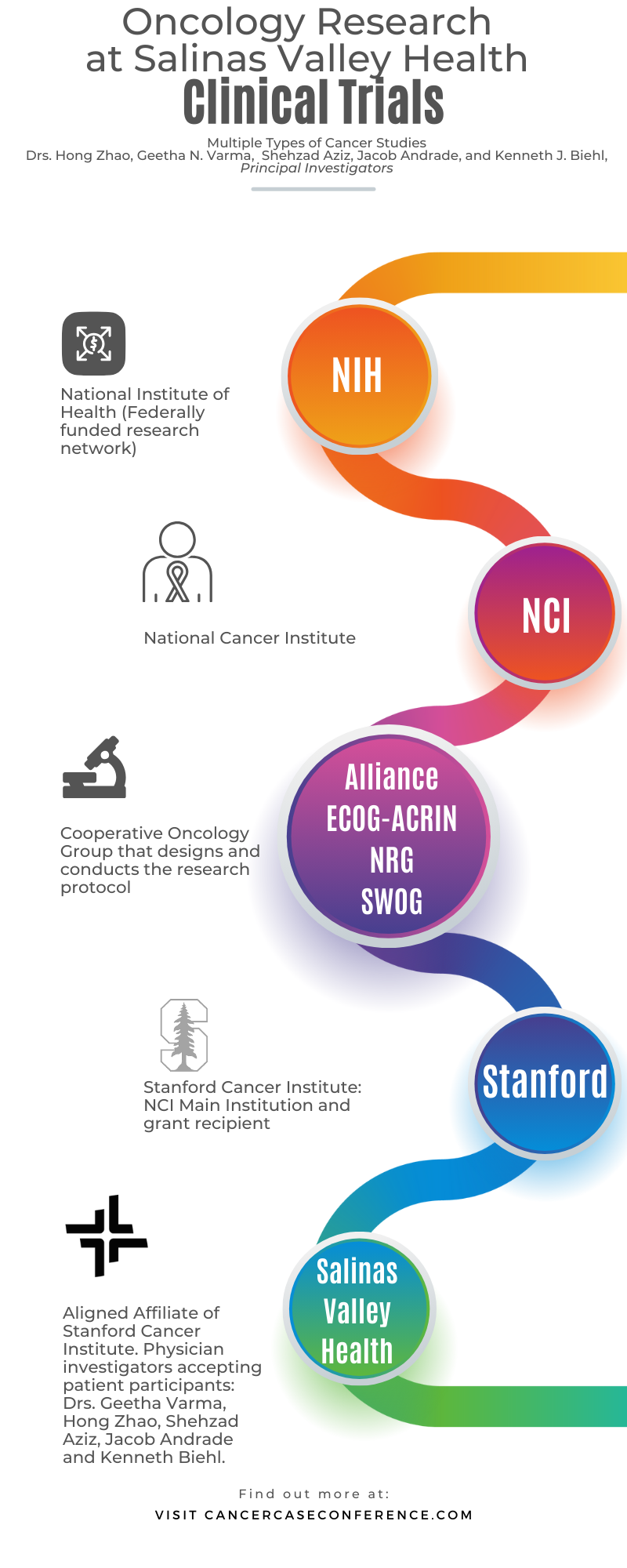
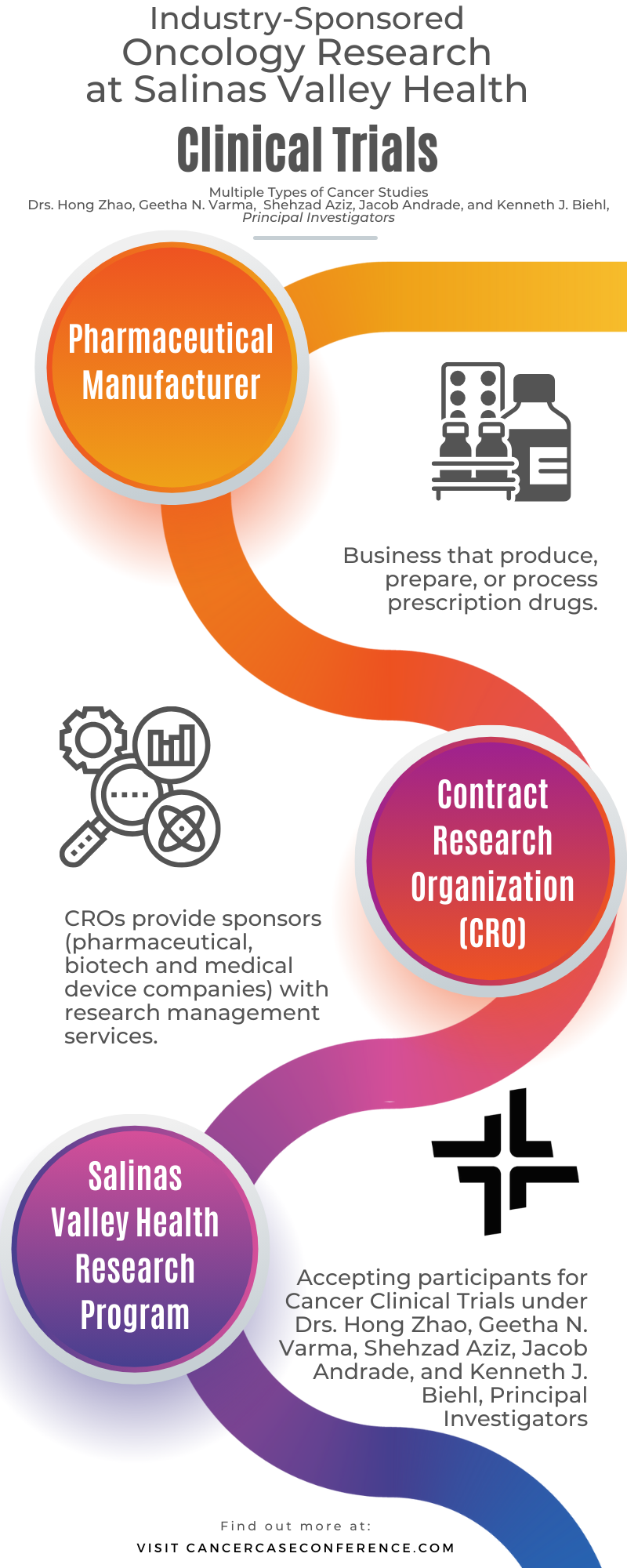
The goal of the Salinas Valley Health Oncology (Cancer) Research Program is to improve patient health and care by providing access to the latest forms of cancer diagnosis and treatment. This is achieved through a diverse range of oncology research studies that align with the types of cancer seen in the patient population.
Cancer Clinical Trials
Clinical trials offer cancer patients the opportunity to test new, investigational treatments under the oversight of the Food and Drug Administration (FDA). These trials aim to advance our understanding of cancer prevention, diagnosis, and treatment. Many of today's FDA-approved cancer treatments were developed from the results of past clinical trials. Participation in a clinical trial is completely voluntary and requires meeting specific eligibility criteria, such as age, health status, and laboratory results.
|
*Note: The National Comprehensive Cancer Network (NCCN) believes that the best management of any patient with cancer is in a clinical trial. Participation in clinical trials is highly encouraged. |
Meet the Investigators
- Geetha N. Varma, MD - Cancer Research Medical Director
- Hong Zhao, MD
- Shehzad Aziz, MD
- Jacob Andrade, MD
- Kenneth J. Biehl, MD
Next Steps
If you or a family member are thinking about being in a research study, feel free to download one of our brochures:
- Become a Research Volunteer: It’s Your Decision (English)
- Ser Voluntario en Estudios Clínicos: Es Su Decisión (Spanish)
- Are You Thinking About Being in Research Study? (English)
- Comprehensive Cancer Center Clinical Trials (for treatment of people with cancer) (English)
- Video: Comprehensive Cancer Center Clinical Trials (for treatment of people with cancer) (English)
- Video: Comprehensive Cancer Center Ensayos clínicos(Para tratar a personas que padecen de cáncer) (Spanish)
- Comprehensive Cancer Center Ensayos clínicos(Para tratar a personas que padecen de cáncer)(Spanish)
- Questions to ask your doctor (English)
- Preguntas para hacerle a su médico (Spanish)
- Deciding to Participate in Clinical Trials (YouTube)
If you have questions about the Oncology/Cancer studies happening at Salinas Valley Health or want more information about our research programs, email us at research@SalinasValleyHealth.com.

